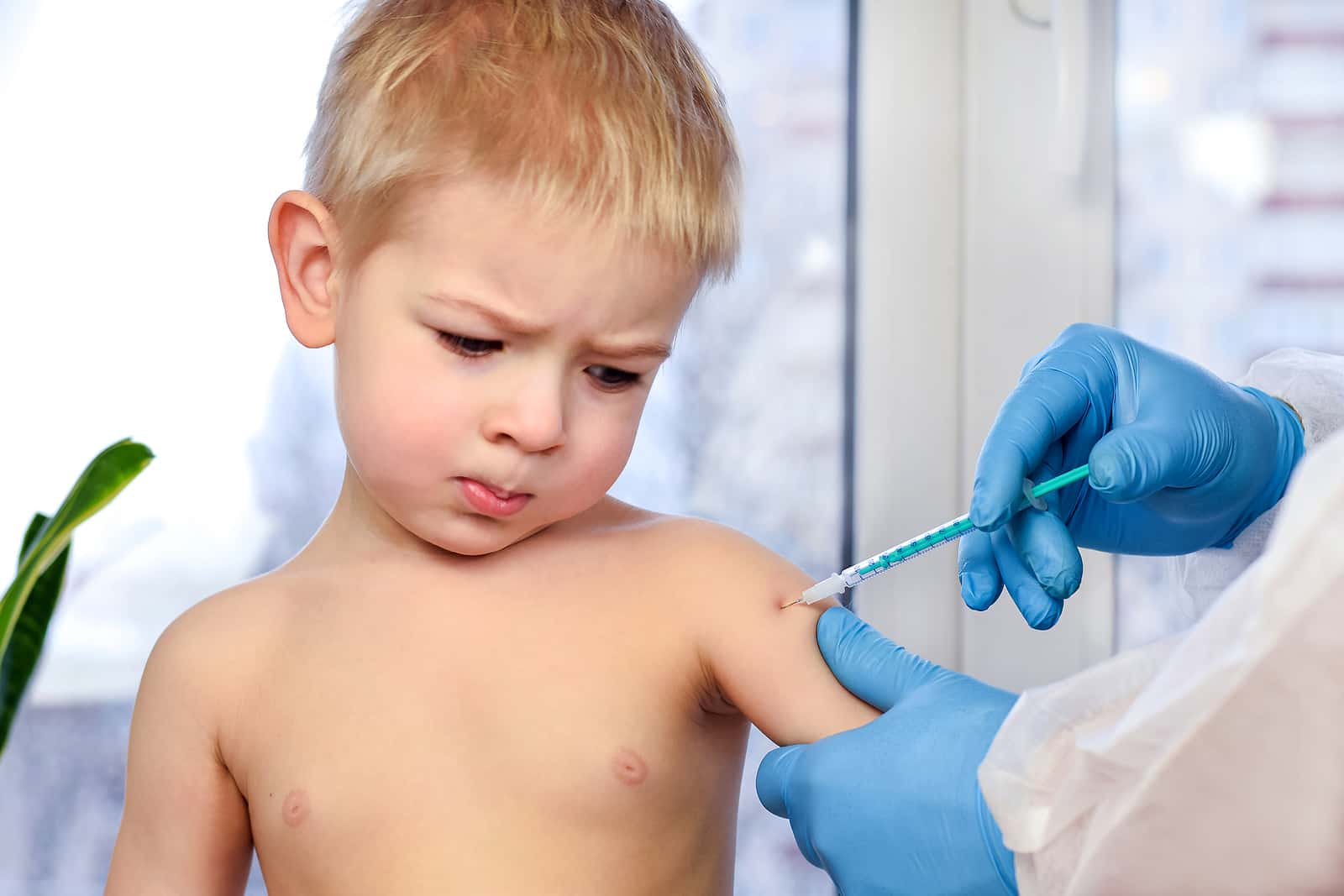
Back at the end of 2020, the FDA issued emergency use authorization for the COVID-19 vaccine from Pfizer-BioNTech. Initially they designated it to protect people 16 years old and older. Later, they extended the authorization to include use in tweens and teens 12 or above. Others followed, and Americans could choose between Pfizer-BioNTech, Moderna and Johnson & Johnson. In August 2021, the agency officially approved the Pfizer-BioNTech vaccine. At the end of October 2021, the agency issued emergency use authorization of a lower dose of this vaccine for youngsters between 5 and 11. But that still leaves the youngest members of the family without a vaccine. Many parents are pleased that the FDA has now been asked to consider emergency authorization of COVID vaccines for little children under 5.
Has Pfizer Studied COVID Vaccines for Little Children?
The request from Pfizer-BioNTech is unprecedented because the trials of COVID vaccine for this age group are incomplete. Although the vaccine is effective in babies from six months to two years old, it is not as effective for youngsters between two and five. Pfizer is currently conducting trials of a third dose in this age group. The company hopes that will bring the immune response up to speed.
If the FDA waits until the drug companies complete the trials, babies and preschoolers will still have no protection. Instead, federal regulators want to give parents a chance to vaccinate these little ones sooner. The idea is that even partial protection is better than no protection. FDA scientists who have reviewed the initial data say the vaccines appear quite safe for little children.
Do Youngsters Need Vaccines?
When the pandemic began, it appeared that children were much less likely than adults to need hospitalization due to COVID-19 infection. While children are still more resilient than adults, youngsters can get quite ill from COVID. Moreover, they may also suffer post-acute complications, such as MIS-C (multisystem inflammatory syndrome in children).
Dr. Paul Offit, Director of the Vaccine Education Center at Children’s Hospital of Philadelphia, told the Washington Post that children can still “suffer and be hospitalized and die from this virus.” Nonetheless, Dr. Offit is not enthusiastic about authorizing an ineffective vaccine. He wants to see proof that the vaccine works. Since he serves on the FDA’s advisory panel for vaccines, his opinion carries weight. Surgeon General Vivek Murthy reassured parents in a White House briefing that COVID vaccines for little children will receive the same rigorous review as the vaccines for older people.

