
We are always surprised and disheartened when health professionals are unfamiliar with the concept of authorized generic drugs. We shouldn’t be. Medical and pharmacy students are not taught that there even are such things. For the most part, health professionals are told that generic drugs are the same as brand name drugs. The FDA has promoted that message for years. That’s why most pharmacies purchase the least expensive generic drugs they can find rather than fill prescriptions with authorized generic drugs that are a bit more expensive. After all, why spend more if the FDA says even the cheapest FDA-approved generic drugs are identical to brand name medicines?
We Are NOT Talking About Brand Name Drugs!
Are you somewhat confused about the idea of authorized generic drugs? We would not blame you. Most people, including most health professionals, believe that there are two categories of medications: brand name pharmaceuticals and generic copycats.
Brands are under patent and can be incredibly expensive. Even after they lose their patent, the price can still be outrageous. For example, 30 tablets of brand name Viagra (100 mg) would cost around $1,900 for a month’s supply, according to GoodRx. The generic version, sildenafil, would run around $20 with a GoodRx coupon. Those prices were as of October, 2023. They will likely change, but for now, you can see the extraordinary difference between a brand name and a generic drug.
But wait…there is a third category of drugs that very few people are aware of. It is Authorized Generic (AG) Drugs. These are exact copies of the brand name medication. They must use the same ingredients and formulate the AG product in a way approved by the brand name company. The company that makes the authorized product usually has to pay the brand name manufacturer for the rights to make and sell the AG version. More about that shortly.
The Big Lie:
For years the FDA has maintained that:
“A generic drug is a medication created to be the same as an existing approved brand-name drug in dosage form, safety, strength, route of administration, quality, and performance characteristics.”
The key words are “the same as.” How would you define the same as? We suspect that many people would conclude that if something is the same as something else it would be identical.
That is not the way the FDA defines “the same as.” For one thing, the FDA allows the pills to use different “inactive” ingredients. That means different fillers, binders, coloring agents and even a different formulation or release mechanism.
When a brand name pharmaceutical loses its patent, other manufacturers can attempt to copy that medication. They have to reverse engineer it, though. That’s because the company that created the original product does not have to share any proprietary information with generic manufacturers. The original manufacturer may also continue to hold a patent on the formulation technology, even if it loses the patent on the chemical compound, known as an active pharmaceutical ingredient (API).
The FDA requires bioequivalence data showing that the generic drug is absorbed in a close approximation to the brand name drug. But the blood levels of the generic do not have to be identical to the original medicine.
The Budeprion XL 300 Debacle:
One example of reverse-engineering involved the antidepressant Wellbutrin XL 300. A generic manufacturer wanted to bring out a similar timed-release version. Although the patent on the API had expired, there was still an active patent on the membrane technology that allowed the active ingredient, bupropion, to be absorbed slowly into the body.
The generic manufacturer had to come up with a different release process. It chose a matrix formulation. The drug was not absorbed in a similar manner as the original brand name medicine.
Patients who were switched from Wellbutrin to the generic version complained of symptoms such as dizziness, nausea, headaches, insomnia, tremor, irritability and digestive problems. In addition, some found that their depression returned.
We badgered the FDA for five years before the agency admitted that the generic Budeprion XL 300 was not “bioequivalent” to the brand name Wellbutrin XL 300. Here is a link to that sad saga in the FDA approval process.
Popular Generic Antidepressant Recalled
Even though Budeprion XL 300 disappeared from the market, Budeprion XL 150 remains. The FDA considers it “the same as” the brand name Wellbutrin XL 150. Take a look at this graph comparing absorption of the generic formulation (Budeprion XL 150) to the brand name antidepressant Wellbutrin XL 150.
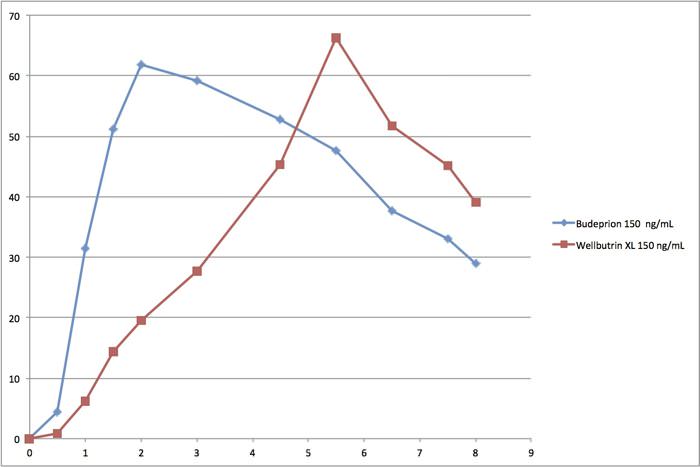
The generic formulation (Budeprion XL 150, aka bupropion) reaches peak blood levels in less than 2 hours (blue line). The brand name Wellbutrin XL 150 doesn’t reach peak blood levels until nearly six hours after it was swallowed. The FDA maintains that the generic form of bupropion has “performance characteristics” that are “the same as” the brand name, even though they are absorbed into the bloodstream quite differently. But that may not dovetail with how patients feel.
A reader shared this experience regarding generic bupropion with us:
“When my manufacturer of this generic [antidepressant] drug was changed, I started having massive PVCs [premature ventricular contractions]. When I went back on my original manufacturer of this drug, the PVCs stopped! The pill that causes me to have PVCs is imprinted with: A 101.”
Another reader offered this:
“Are you still running tests on generic bupropion? I have a feeling mine (150XL) (A101) is not working properly. I noticed a significant change in mood and return of symptoms after being switched to it from a different 150XL generic.”
The FDA has not responded yet to our concerns about this situation.
What About Authorized Generic Drugs?
According to the FDA, authorized generic drugs are exactly the same as the brand name. The only difference is that they don’t have the brand name on the label. Sometimes they are even made on the same production line as the brand.
Please note the subtle difference in wording. The FDA says that generic drugs are “the same as” brand name drugs. The FDA states that authorized generic drugs are :
“…the exact same drug product as the branded product.”
An Analogy:
We will let you appreciate that subtle but significant difference in wording.
Here’s one way to think about it. Coca-Cola is the brand name beverage you are familiar with. If another company tried to duplicate Coke, it would have to reverse engineer it. The best the generic manufacturer could do is call its beverage a cola soft drink. It might be similar but not identical to the original formula Coca-Cola.
If the Coca-Cola company did a deal with a generic manufacturer of soft drinks and gave that company the secret formula, this “authorized generic ” cola version would be the exact same product as Coke without the brand name on the bottle. It might even be made on the Coca-Cola production line.
Of course, the Coca-Cola company has no incentive to do such a deal. But when brand name drug companies lose a patent on a medicine, they do have an incentive to continue to compete with other generic drug manufacturers. That is why they sometimes allow other companies (and sometimes their own subsidiary companies) to sell authorized generic products that are indeed identical.
Drug Scandals:
Because of scandals in foreign manufacturing and the lack of FDA oversight during the pandemic, some patients have become suspicious of generic drug quality. Others have experienced therapeutic failures or side effects from “standard” generics. That’s why they have been seeking authorized generic drugs. That has aggravated some pharmacists.
Can Your Pharmacist Fill Prescriptions with Authorized Generic Drugs?
We have continued to receive complaints about other generic products. One example is metoprolol, a beta blocker heart medicine.
A reader recently asked about this medicine:
“My cardiologist has prescribed generic metoprolol for years. I had a heart attack in 2016 and have multiple heart conditions.
“I recently noticed that my prescription pills drastically changed appearance from small white tabs to medium-large over-stuffed colored pills. Then I realized that the manufacturer’s name was different. I became suspicious, as I have read in my natural health newsletter that some medications from outside the country may contain dangerous fillers.
“When I returned to the pharmacy and asked for my previous prescription, they said that they no longer carry that one. Neither did the other drugstores in my community. All the pharmacists asked me what I meant by “authorized generic”!
“In the meantime, I wrote a personal request to my cardiologist through my health portal to please prescribe an authorized generic for metoprolol. I mentioned to him that the FDA has recalled some versions of metoprolol and that I need his assistance for my heart’s sake.
“The next day, I asked the pharmacist if he could help me find authorized generic metoprolol. He refused.
“My next prescription of metoprolol is almost due. The pharmacy will give me pills by this same questionable manufacturer. Should I just risk my heart by taking a drug I don’t trust? What else can I do?”
Authorized generic drugs are produced with the same formula, sometimes on the same production line as the brand name medicine. In the case of metoprolol, an authorized generic is available from EaglePharmacy.com in Lakeland, Florida. We have NO relationship with Eagle Pharmacy! Cardiologist, Harry Lever, formerly of the Cleveland Clinic, has been concerned about generic metoprolol for years. He alerted us that Eagle Pharmacy sells Toprol AG (the authorized generic metoprolol).
Another Metoprolol Story:
Lew tested immediate release metoprolol against the extended release (ER) generic version. His experiment is very revealing. That’s because a beta blocker like metoprolol should always show the heart rate if it is working correctly.
Here is what he says:
“My PCP switched me from 25mg 2x a day immediate release to 50mg ER metoprolol once a day. I was having high blood pressure readings in the morning. Was told to take ER tab at bedtime.
“Everything was fine for about 2 weeks with the ER version. Then about 10:30 one morning I had a rapid heart rate (110-120) and high blood pressure 160/79. It gradually dissipated during the day. By the time I spoke with my PA by 15:00, things were normal.
“The next morning, 04:30 it happened: rapid rate, forceful and some PVC-type arrhythmias. Decided to go to the ER; thought maybe they could capture event on a ECG.
“ECG was negative by then, but heart rate was around 105 and BP 180/85. Was there way too long; they had several other emergencies, but when I left 6 hours later, BP 140/79 and heart rate 100.
“After getting home I decided to go back to the immediate release version of metoprolol. No problems for next three days. So, thought I’d return to extended (ER) version. Maybe just a fluke.
“I was wrong. The rapid heart rate and arrhythmias symptoms returned early next morning. I took 12.5 mg immediate release, which settled it down in about 45 minutes. Seeing that my doc appt was about 16 days off, I decided to go back to taking the immediate rls version.
“I did so for 12 days; no problems during that time, things were great. However, just to prove to myself one more time, I changed to the ER version. Two days later the symptoms were back. Fortunately, I had plenty of the old immediate release pills left.
“I am not a big believer is coincidences, especially three times. But, as far as I’m concerned it is the extended-release generic metoprolol that is the cause of my issues. I wonder if there may be too many manufacturers now and too little oversight by the FDA.”
This reader wants to know why most pharmacists won’t fill prescriptions with authorized generic drugs:
Q. You have written about “authorized generic” drugs. I can’t find a pharmacy that will fill prescriptions with authorized generic drugs. Can you steer me in the right direction?
A. The makers of authorized generic drugs have negotiated agreements with the brand-name manufacturers so that they can use the exact same “recipe.” Sometimes authorized generic drugs are even made on the same production line as the original brand name medicine.
Reverse-Engineering Generic Drugs:
“Regular” generic drugs are often reverse engineered. That is to say, the manufacturer tries to figure out how the brand name company created the original medicine. That information is considered proprietary.
In her best-selling book, Bottle of Lies, Katherine Eban describes the process of reverse-engineering:
“A brand-name drug, no matter how complex or difficult to make, inevitably follows a recipe, such as mix fifteen minutes, granulate, mist until ingredients reach 4 percent moisture content, mix again for thirty minutes. Making a generic version, however, requires figuring out a different recipe, ideally one that is faster to make but produces a similar result. That effort of reverse-engineering is undertaken by process chemists.”
Katherine goes on to describe Rajiv Malik, a process chemist who had worked at the Indian drug company, Ranbaxy. Rajiv was head of formulation development and regulatory affairs. He had mastered the art of reverse-engineering generic drugs based on the brand name formulation.
The book describes Rajiv’s effort to copy the brand name acne drug Accutane. Ranbaxy was planning to market it under the name Sotret. There was only one problem. As production ramped up, the generic drug wasn’t dissolving properly.
Katherine describes what happened next:
“Given the circumstances, the FDA’s regulations required Ranbaxy executives to withdraw the drug from the market and suspend making it until the failures could be remedied…
“The push for profits won out. They chose to continue the launch and conceal the problems from regulators, even as they returned to the laboratory in search of a solution.”
Why Don’t Pharmacies Fill Prescriptions with Authorized Generic Drugs?
Once upon a time, independent pharmacists could decide which generic drugs they would stock. Some independent pharmacists still do that. But these days most chain pharmacies, big-box pharmacies, mail-order pharmacies and grocery pharmacies have central buying departments.
They may also use PBMs (pharmacy benefit managers). These organizations cut deals with generic drug companies. When you ask your local chain pharmacist to stock an authorized generic (AG) drug, the chances are good that she will look at you with dismay. That’s because she has little control over what generic drugs are placed on the pharmacy shelves. Sometimes a pharmacist will become upset with a request to fill prescriptions with authorized generic drugs.
This pharmacist lost it when asked to fill prescriptions with authorized generic drugs:
“After reading your newspaper column about authorized generic drugs I had to fill a prescription. I saw the pharmacist at my local chain drugstore and asked him about filling it with an authorized generic. I even mentioned the article in JAMA Internal Medicine (Jan. 25, 2021) that was in your column.
“He blasted me so angrily. He insisted that all of their prescriptions, including generics, are authorized by the U.S. government. Nothing on the web has any validity, including the article in JAMA Internal Medicine. End of message. Wow! His limit had been maxed.”
Another pharmacist brought up the problem of insurance:
“As a pharmacist for over 40 years I can tell you the problem is most likely due to insurance reimbursement. Insurers will quickly latch onto the cheapest generic available, authorized or not, and reimburse according.
“Keep in mind that all generics are approved by the FDA. Authorized generics simply have the stamp of approval from the brand manufacturer, most likely due to a financial agreement.
“Concerta-brand methylphenidate (36 mg) costs a pharmacy around $1200/100. The authorized generic from Patriot is around $800/100. A regular generic version costs around $125/100. The problem is to get the insurer to pay for the authorized generic, which requires a physician intervention to argue the case with the insurer.
“Pharmacists are caught in the crossfire with the certainty that filling the authorized generic will result in a substantial financial loss weighed against the patient needs. It’s not that they simply don’t want to take the time to order the authorized generic.”
Can Pharmacies Fill Prescriptions with Authorized Generic Drugs?
Pharmacists are incredibly busy. They barely have time to fill “regular” prescriptions. Asking them to find out who makes a particular authorized generic drug is time consuming. Then getting a pharmacy chain buyer to order it is even more problematic.
Finding a list of authorized generic drugs can also be a challenge, and we don’t know of any list that is complete. However, you will find the best list we could assemble on our website at this link. You can also find other ways to access affordable brand name drugs in our eGuide to Saving Money on Medicines. Look for it in the Health eGuides section of the website.
Not all medications have an authorized generic available. If you find the drug you are looking for, you will need to ask the pharmacist to order from that manufacturer specifically. Independent pharmacies may be more willing to do this than chains.

