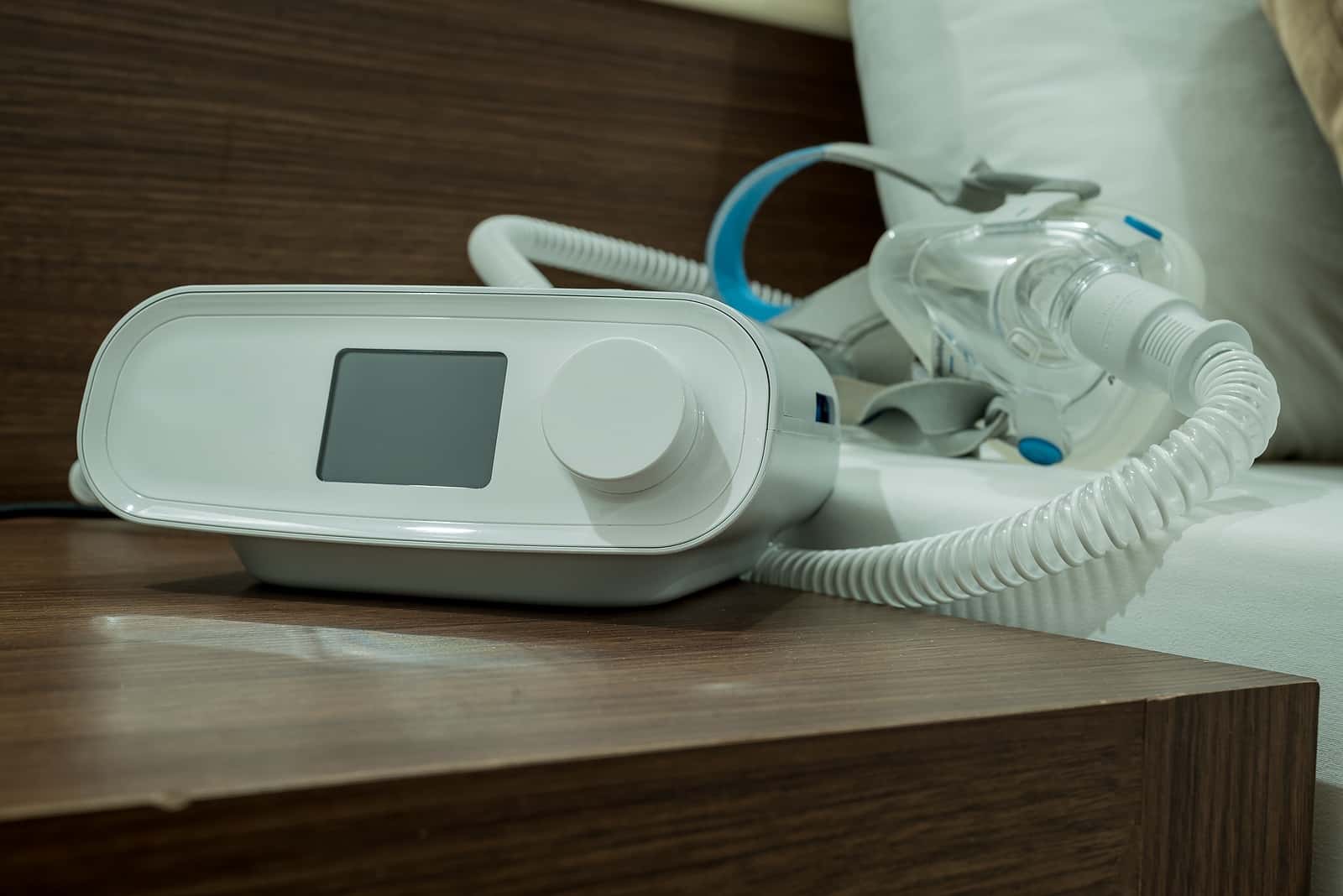
According to the AMA, “About 30 million people in the United States have sleep apnea, but only 6 million are diagnosed with the condition.” People with sleep apnea may stop breathing many times during the night. These episodes can last a few seconds or as long as a minute or two. They can occur dozens of times an hour. People with sleep apnea usually treat this condition with a machine providing continuous positive airway pressure (CPAP). An investigative report from ProPublica and the Pittsburgh Post-Gazette (Sept. 27, 2023) reveals a startling CPAP crisis that took years to fully uncover. On January 31, 2024 the FDA updated its medical device report (MDR) to acknowledge 561 deaths associated with Philips CPAP and BiPAP machines.
What’s the CPAP Crisis Actually About?
One of the major manufacturers is in trouble with the FDA. The sound-dampening foam used in its design has a tendency to break down and emit fumes and hazardous particles into the air it is pushing into patients’ airways.
That problem is bad enough, but to make things worse, Philips Respironics apparently failed to warn customers about the danger. An investigation by ProPublica and the Pittsburgh Post-Dispatch (Sept. 27, 2023) showed that the company had received at least 3700 complaints over more than a decade before taking decisive action in 2021. The FDA is critical of the company’s response and has expressed skepticism that safety tests of the foam were adequate.
In fact, according to the FDA:
“Since April 2021, the FDA has received more than 116,000 MDRs, including 561 reports of death, reportedly associated with the PE-PUR foam breakdown or suspected foam breakdown.”
Philips Will Fix its Problems Before Selling More Machines:
Now Philips, the biggest maker of such CPAP machines, will not be selling them in the US. The Dutch manufacturer has recalled millions of breathing devices and now will not sell new Respironics devices until it has made required changes in its manufacturing process. This is likely to take years.
As we noted above, the problem with the recalled machines was sound-dampening foam that deteriorated and could be inhaled. This posed a possible cancer risk. Although Philips will not be selling new CPAP or BiPAP machines in the US until it has met all requirements in the consent decree it reached with the FDA, it is allowed to provide customer support and replacement components for machines people already own.
What’s It Like To Suffer Sleep Apnea?
I have tried holding my breath while timing it with my smart phone stopwatch. It starts to get uncomfortable around 25 seconds and I have to take a breath around 30 seconds. I have a hard time imagining what it would be like to go longer than that.
Obstructive sleep apnea (OSA) is caused by the collapse of muscles in the throat. This can block the airway. The result is reduced oxygenation of the circulating blood.
Patients with OSA often suffer from daytime sleepiness and brain fog. That makes them more prone to accidents. They are also more likely to develop hypertension, strokes, irregular heart rhythms or heart attacks.
Symptoms can include noisy snoring interrupted by gasping or gagging sounds. For a bed partner, this can be scary and/or annoying. It’s hard to sleep when the person nearby is “sawing wood” and intermittently gasping for air. People with OSA may also complain about frequent nighttime trips to the bathroom to pee, morning headaches, daytime irritability and “cotton mouth” upon awakening.
Treating OSA and the CPAP Crisis:
To treat sleep apnea, doctors often prescribe machines that pump air in a continuous stream that can help keep the airways open. They are called CPAP devices, for continuous positive airway pressure. Some people find the devices noisy and uncomfortable. You have to wear a kind of face mask that pushes air into the throat and lungs.
Others find the devices improve the quality of their lives. Here is just one of the hundreds of messages we have received on our website:
Jerry reports that CPAP made a difference:
“Sleep apnea is a medical condition that can cause frequent nighttime urination. After being diagnosed with severe obstructive sleep apnea, I was treated using CPAP therapy.
“The very first night after getting my CPAP machine I slept for a full 7 hours. Before treatment, I was waking and passing large amounts of urine every 1-2 hours. My blood pressure went from high to normal, and my heart rate during exercise dropped by 20 heartbeats.”
The CPAP Crisis Is Creating Chaos:
Needless to say, people with serious sleep apnea rely on these machines and are well aware that their lives depend on them. Just imagine the panic they might feel if their machines were faulty or no longer available.
That has happened to far too many patients over the last few years, as the company that dominates the industry, Philips Respironics, fumbled a recall of faulty CPAP devices.
Here is what one reader wrote us about the CPAP crisis:
“My husband has sleep apnea, so he has used a CPAP for over 12 years. Recently his machine stopped working. When he contacted his supplier, he was told his machine had been recalled and he would have to wait for a replacement. They advised him to contact the manufacturer, Philips.
“He called Philips, and after following the instructions to restart the machine, was told it was not working. That was July 11. We had just gotten home from the ER where we both tested positive for COVID and received infusions. So he has been without his CPAP since then.
Anxiety interferes with sleep:
“He is very aware of the dangers of not using the CPAP machine and has been sleeping very uncomfortably since then. I have been anxious as well, just listening to his snoring. Here’s hoping he does not stop breathing, as he used to do before getting the CPAP.
“To my mind, this situation is similar to not having baby formula available. Doctors warn that using the machine every night is of critical importance. What can be done to help so many people in need? I am hoping that sleep apnea patients can soon get the machines they desperately need for a good safe night’s sleep.”
How Has the FDA Fumbled the CPAP Crisis?
Our reader is right to compare the situation with CPAP machines to the old baby formula shortage. Both resulted from the FDA’s inadequate oversight. In our opinion, the FDA has fumbled a few too many oversight responsibilities.
That’s not just our opinion. An article in JAMA Internal Medicine, July 26, 2021 reviewed the FDA’s oversight of MAUDE (Manufacturer and User Facility Device Experience). The agency relies on device manufacturers, distributors, physicians, patients, hospitals and other health care facilities to submit reports of problems. Unfortunately, the FDA pays much of its attention to deaths, rather than all serious complaints.
The analysis in JAMA Internal Medicine points out that:
“For the overall sample, the percentage of reports with deaths that were not classified as deaths was 23%, suggesting that approximately 31,552 reports in our sample had deaths that were classified in other categories.”
If the FDA ignores serious complaints and overlooks deaths with misleading codes, it risks leaving flawed medical devices on the market long past their “use by” date. You can read more about the agency’s fumbling and bumbling at this link.
What Went Wrong With CPAP Machines”
On July 29, 2021, the FDA issued an announcement about problems with Philips Respironics BiPap and CPAP machines.
On May 19, 2022 the agency updated its warning:
“Philips Respironics (Philips) voluntarily recalled certain ventilators, bi-level positive airway pressure (also known as Bilevel PAP, BiPAP, or BPAP) machines, and continuous positive airway pressure (CPAP) machines in June 2021 due to potential health risks. The polyester-based polyurethane (PE-PUR) foam used in these medical devices to lessen sound and vibration can break down. If the foam breaks down, black pieces of foam, or certain chemicals that are not visible, could be breathed in or swallowed by the person using the device.”
The Washington Post reported on July 29, 2022 that:
“Today, those machines are at the heart of one of the biggest medical device debacles in decades.”
“If inhaled or swallowed, the emissions could cause headaches, asthma, lung problems and even cancer, the company warned in launching a massive recall. The Food and Drug Administration classified the recall as the most serious type, saying “use of these devices may cause serious injuries or deaths.”
ProPublica and the Pittsburgh Post-Gazette Sued the FDA for Data!
To better understand the history of the CPAP crisis, the nonprofit investigative newsroom ProPublica and the Pittsburgh Post-Gazette filed a suit against the FDA (Sept. 29, 2023):
“…accusing the agency of holding back records related to the sweeping recall of breathing machines that were sold around the world.”
The investigative reporters go on to describe the CPAP crisis:
“After the lawsuit was filed in April, the FDA agreed to begin producing documents. The agency, however, fully redacted the company’s test results and assessments on the degrading foam — more than 1,000 pages — even though the FDA has previously summarized those results in publicly available records.
“The agency cited an exemption in FOIA [Freedom Of Information Act] law that protects trade secrets and commercial or financial information that could impact a company’s business interests.”
Does the FDA Protect the Industry It’s Supposed to Regulate?
We used to believe that the Food and Drug Administration was supposed to protect the American public from unsafe pharmaceuticals and devices. But for far too long we have worried that the agency is working hard to protect the industries it is supposed to regulate.
Take generic drug companies, for example. The FDA has found serious problems with cleanliness, manufacturing processes and actual fraud. When it inspects a pharmaceutical firm and finds serious problems, the agency sends a 483 letter to the company describing the nature of the violations. You would think that the FDA would want the public and health professionals to know about the problems that were uncovered. Far too often, in our opinion, the watchdog “redacts” important information about infractions. That drives me nuts!
Senator Richard Blumenthal is Upset About the CPAP Crisis!
On October 11, 2023 ProPublica updated its investigative report about the CPAP crisis:
“Sen. Richard Blumenthal, D-Conn., has expanded his call to take action against medical device powerhouse Philips Respironics, sending a letter to federal regulators demanding aggressive enforcement against the company for withholding thousands of warnings about a dangerous defect in its breathing machines.
“In the letter on Tuesday to Food and Drug Administration Commissioner Robert M. Califf and Attorney General Merrick Garland, Blumenthal cited a ProPublica and Pittsburgh Post-Gazette investigation last month that revealed the company sold millions of sleep apnea machines and ventilators even after finding that an industrial foam placed inside them was breaking down and emitting chemicals at dangerous levels.”
“A yearlong investigation by the news organizations found that Philips kept secret more than 3,700 complaints about the faulty devices over the course of 11 years before launching a massive recall.
Over a year ago the Washington Post reported:
“In May, the FDA announced it had received 21,000 reports, including 124 deaths, concerning the breakdown of the polyester-based polyurethane foam in sleep apnea machines and ventilators during the past year — a sharp increase from 30 the previous decade.”
How long has the maker of CPAP machines known there was a problem? Why didn’t the FDA discover this problem on its own? What should it do about the CPAP crisis? We are critical of the way the FDA handled this situation.
Some Recommendations from The People’s Pharmacy:
Here are some of our suggestions:
- 1) The FDA should be more proactive regarding critical medical devices so that life-threatening problems of this sort never happen again.
- 2) The FDA should make their investigations much more transparent. Protecting drug companies or device manufacturers by citing a clause in the Freedom of Information Act (FOIA) about “commercial” information is a cop out. The public deserves to know what violations or infractions the agency discovers during its investigations!
- 3) The companies that screw up should be punished severely! Patients should be reimbursed for any harm that is caused by faulty drugs or devices. We also think that corporate leadership should be punished for serious violations or infractions. That might make companies a tad more careful going forward.
What Do You Think?
We would love to read your thoughts about the CPAP crisis in the comment section below. Do you know someone who snores and has obstructive sleep apnea? Have they ever used a CPAP machine? Has their device been recalled? What are they doing now? Do you think the FDA has done a good job? Does the agency need to be more proactive when it comes to protecting the public health?
If you think this article has merit, please send it to friends and family. We suspect that someone you know snores, has sleep apnea and/or has a CPAP-type machine. They (and their health care providers) may not know about the problems with these devices. It’s super easy to share. Just scroll to the top of the page and click on the icons for email, X (Twitter) and Facebook.
While you are at it, please encourage your contacts to sign up for our free online newsletter. You may have noticed that Google accepts a lot of drug and device ads. Is it any wonder that articles like this disappear almost without a trace? The only way your acquaintances can read our independent voice is to subscribe to our newsletter at this link. Thank you for supporting our work!
Citations
- Lalani, C., et al, "Reporting of Death in US Food and Drug Administration Medical Device Adverse Event Reports in Categories Other Than Death," JAMA Internal Medicine, Sept. 1, 2021, doi: 10.1001/jamainternmed.2021.3942
- Salant, J.D. "U.S. Senator Expands Call for Crackdown on Philips Respironics," Pittsburgh Post Gazette & ProPublica," Oct. 11, 2023

